Research Portfolio
-

Organisational Principles of Spatial Microbial Communities
Bacteria primarily exist within dense communities composed of diverse genotypes that interact with each other. Ecological interactions between cells or populations can govern cellular organisation and growth properties within communities, ultimately influencing the contribution of communities in key ecosystem processes like polysaccharide degradation.
In order to develop a quantitative understanding of organisational as well as functional dynamics, I study simple communities composed of bacterial species that interact synergistically or antagonistically with each other. I study the assembly dynamics of individual cells within distinct communities in microfluidic growth devices coupled to automated time-lapse microscopy. Such an approach allows the measurement of growth properties at the level of single cells and building a quantitative framework for studying ecological dynamics at the level of individual cells. First insights from this work reveal that ecological interactions impact spatial organisation of microbial communities, thereby altering functional properties.
-

Microbial collective behaviors
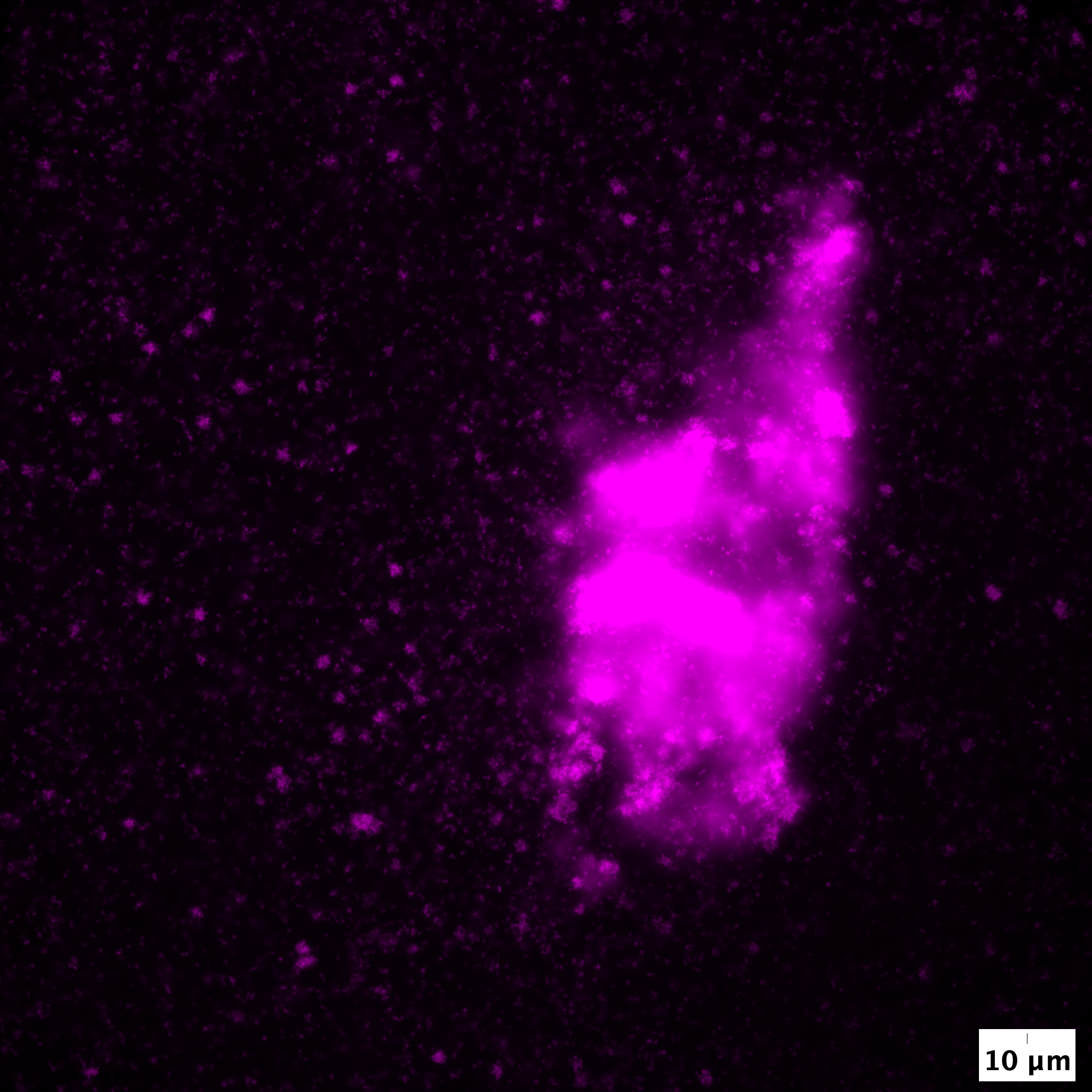
Bacterial cells often transition between collective and solitary growth modes. What ecological factors drive cells to display distinct behaviours? What benefits do collective and solitary growth modes confer to isogenic individuals within a population? Which molecular processes enable cells to transition between distinct growth modes? How do distinct behaviours evolve in microbial populations?
I address these questions using an empirical approach that combines microfluidics coupled to automated time-lapse microscopy, image analysis, genetics, physiological assays, experimental evolution and genomics.
Insights from our recent work on Caulobacter crescentus and diverse marine Vibrionaceae isolates is that cells engage in collective growth states when they have to metabolise complex polysaccharides like xylan or alginate, which represent a large fraction of the organic stock of carbon in nature. Collective growth allows individuals to benefit from each another’s degradative activities and reduce diffusional loss of nutrients. When simpler mono- and oligosaccharides become available, cells increase motility and transition to solitary growth modes since the benefit of a collective is no longer required. Cells can thus rapidly tune their behaviours based on the complexity of the nutrients in their environment.
-
Evolution of dependence and collectivity
Bacterial communities are often composed of diverse genotypes that depend on each other for their metabolic needs. How do these dependencies emerge? Under what conditions do initially autonomous populations evolve metabolic dependencies? How does collectivity arise in bacterial populations enabling cells to grow optimally in complex environments? In order to address these questions, I use a combination of experimental evolution and genomics.
In my PhD, I ellucidated that bacterial populations can rapidly lose biosynthetic functions when they evolve in environments where the function is externally compensated. We do so by performing an evolution experiment where Escherichia coli populations were serially propagated in the presence or absence of amino acids. By reseqeuncing the evolved populations to understand the basis of evolutionary change and comparing growth dynamics to the evolutionary ancestor, we show that the evolution of metabolic dependencies was adaptive. Our study provides strong evidence to show how nutrient environments drive the evolution of metabolic associations in microbial communities. To know more see our paper in Plos Genetics.
Ongoing work: Bacterial populations often display collective behaviours, especially when they have to metabolise complex polysaccharides. To unravel cellular pathways that contribute to the evolution of collectivity, I investigate the genetic changes that ensue in populations of Caulobacter crescentus and marine Vibrionaceae populations that have been experimentally evolved to display aggregative behaviours. In addition to genome sequencing to understand the process of evolutionary change, I employ transcriptomics to unravel cellular pathways that contribute to the emergence of collectivity in microbial populations.
